
Selected Publications
- Choi, K. The structure–property relationships of clinically approved protein kinase inhibitors. Curr. Med. Chem. 2023, 30, 2518-2541.
- Kang, H.S., Kim, H.M., Song, J.E., Kim, M.J. Choi, K. Real-time fluorescence imaging sensor for measuring glutathione in organelle and preparation method therefor. U.S. Patent 11,472,825, 2022.
- Lim, J., Heo, J., Ju, H., Shin, J.-W., Kim, Y., Lee, S., Yu, H.Y., Ryu, C.-M., Yun, H., Song, S., Hong, K.-S., Chung, H.,-M., Kim, H.-R., Roe, J.-S., Choi, K., Kim. I.-G., Jeong, E.M., Shin, D.-M. Glutathione dynamics determine the therapeutic efficacy of mesenchymal stem cells for graft-versus-host disease via CREB1-NRF2 pathway. Sci. Adv. 2020,
6, eaba1334.
- Kim, I.-G., Choi, K., Jeong, E.M., Kang, H.S. Real-time imaging sensor for measuring cellular thiol level. U.S. Patent 10,215,757, 2019.
- Jeong, E.M., Yoon, J.-H., Lim, J., Shin, J.-W., Cho, AY., Heo, J., Lee, K.B., Lee, J.-H., Lee, W.J., Kim, H.-J., Son, Y.H., Lee, S.-J., Cho, S.-Y., Shin, D.-M., Choi, K., Kim. I.-G. Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function. Stem Cell Reports. 2018, 10, 600-614.
- Kaur, M., Ahn, Y.-H., Choi, K., Cho, M.J., Choi, D.H. A bifunctional colorimetric fluorescent probe for Hg2+ and Cu2+ based on a carbazole–pyrimidine conjugate: Chromogenic and fluorogenic recognition on TLC, silica-gel and filter paper. Org. Biomol. Chem. 2015, 13, 7149-7153.
- Khosla, C., Watts, R.E., Siegel, M.J., Choi, K. Transglutaminase inhibitors and methods of use thereof. U.S. Patent 8,871,718, 2014.
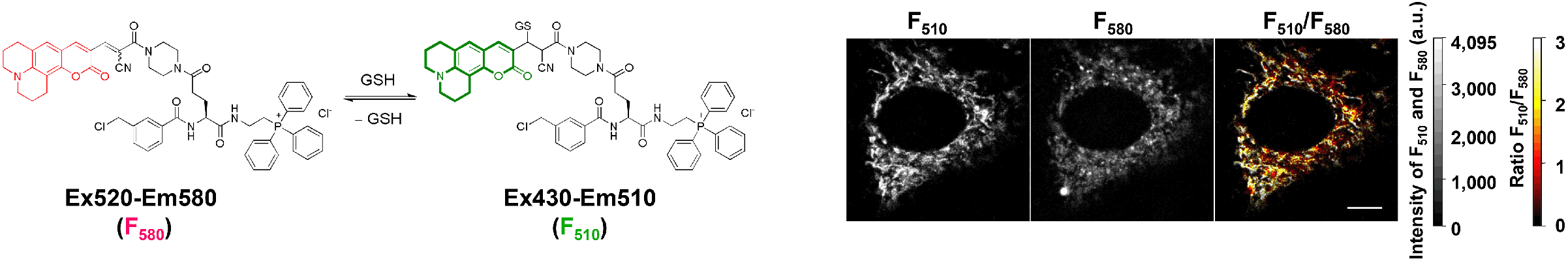
- Cho, AY., Choi, K. A Coumarin-based fluorescence sensor for the reversible detection of thiols. Chem. Lett. 2012, 41, 1611-1612. FreSH
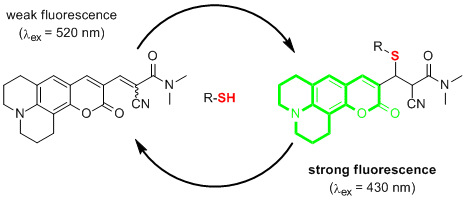
- Kim, S.-M., Choi, K. A practical solvating agent for the chiral NMR discrimination of carboxylic acids. Eur. J. Org. Chem. 2011, 4747-4750.
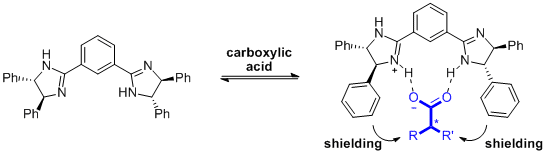
- Han, S.-Y., Choi, K. N-Arylcarbonylpseudoprolines as tunable chiral derivatizing agents for the determination of the absolute configuration of secondary alcohols. Eur. J. Org. Chem. 2011, 2920-2923.
- Shim, Y.-J., Choi, K. Cyclic ketals of tartaric acid: Simple and tunable reagents for the determination of the absolute configuration of primary amines. Org. Lett. 2010, 12, 880-882.
- Park, E.-K., Park, B., Choi, J.-H., Choi, K., Cho, M. Chirality transfer effects in proline-substituted coumarin compounds. J. Phys. Chem. B 2009, 113, 11301-11305.
- Ahn, H.C., Yun, S.-M., Choi, K. A proline-based macrocyclic amide with S4 symmetry. Chem. Lett. 2008, 37, 10-11.
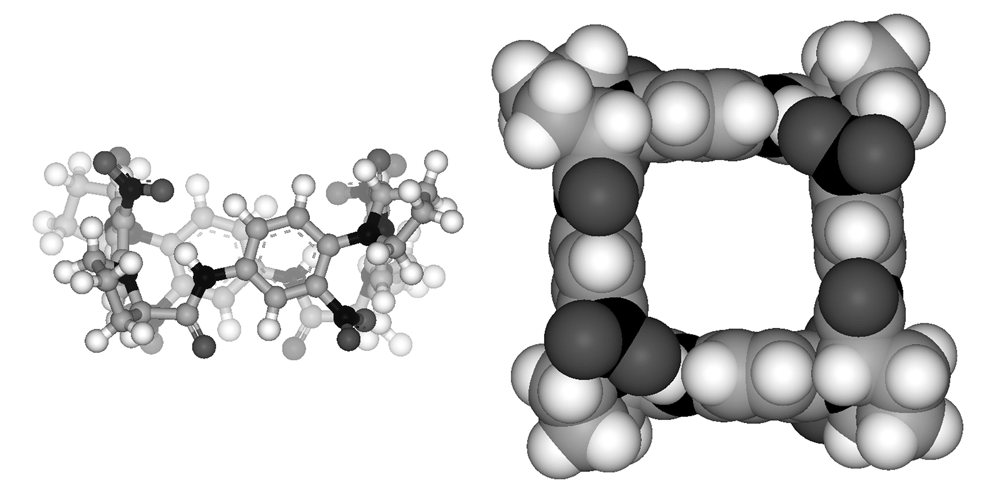
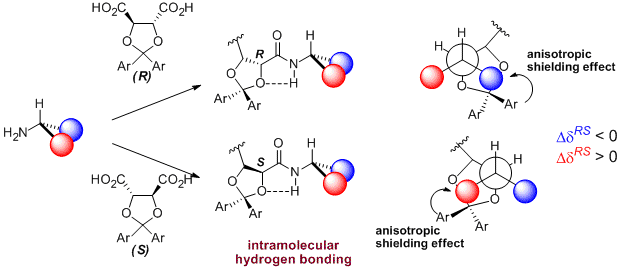
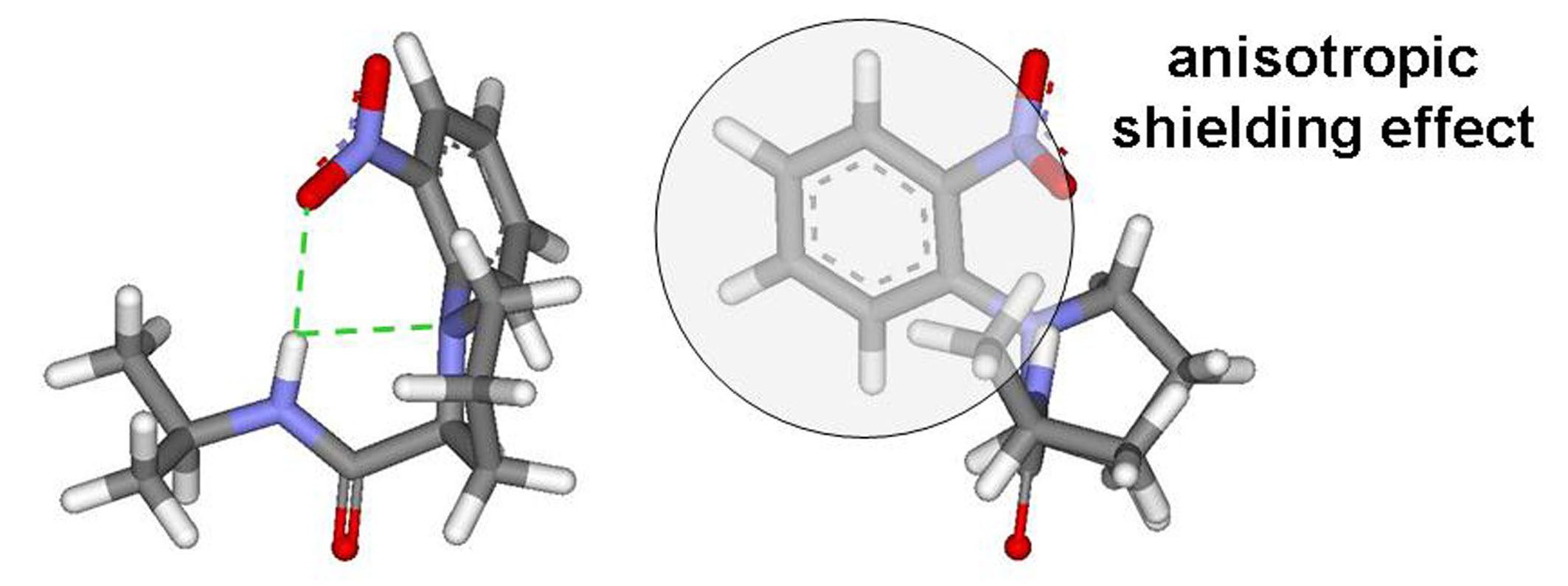
- Ahn, H.C., Choi, K. N-(2-nitrophenyl)proline: An intramolecular hydrogen bond forming reagent for the determination of the absolute configuration of primary amines. Org. Lett. 2007, 9, 3853-3855.
Department of Chemistry, Korea University, 145 Anam-ro Seongbuk-gu, Seoul 02841, Korea (ROK)